Resources
Vaccine Approval in the United States
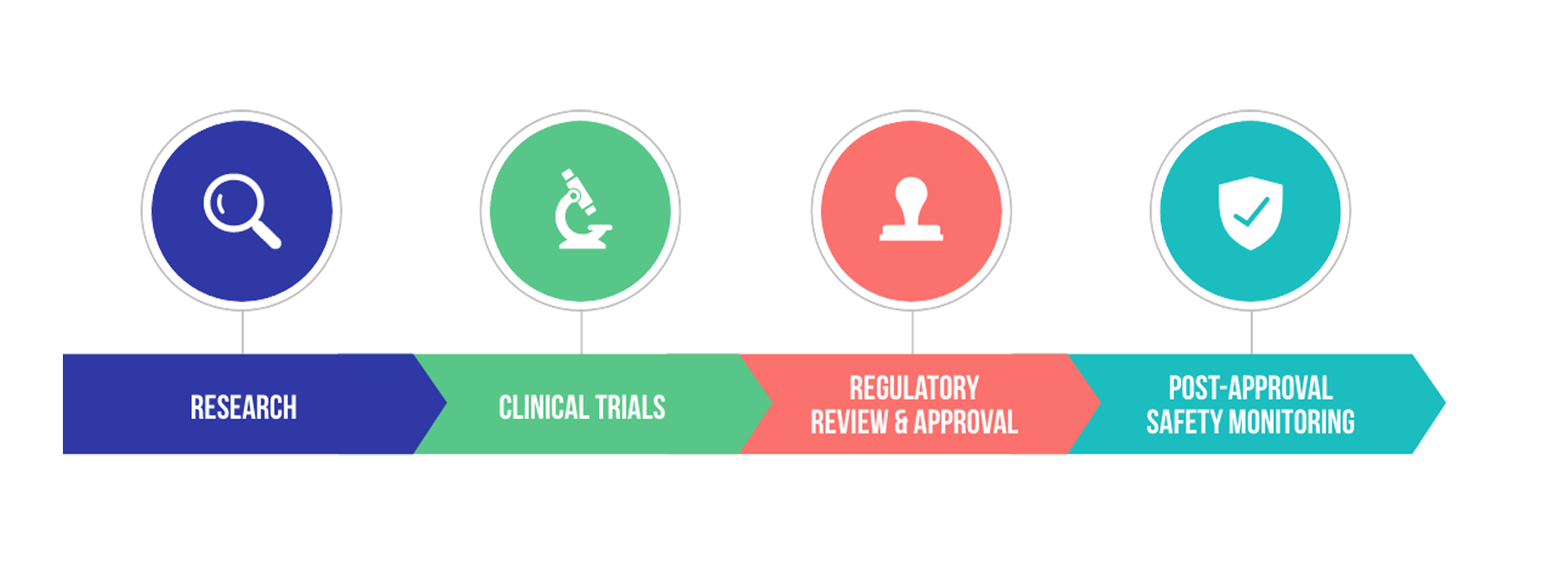
At the FDA, we uphold globally respected standards for product quality, safety, and efficacy. We monitor the entire process of vaccine development from beginning to end and then follow these products after they are made available to the public.
COVID-19 vaccine development is following the FDA’s gold-standard review process that includes research, multi-stage clinical trials, robust regulatory review and approvals, and ongoing safety monitoring once a vaccine becomes publicly available.